Tuberous Sclerosis Complex (TSC) is a genetic disorder that affects people in many different ways. Some people with TSC are so mildly affected they may go through life without the diagnosis being made.
TSC is often referred to simply as Tuberous Sclerosis (TS) and affects approximately 1 in 9000 people. There is no cure for TSC but there are treatments available for many of the symptoms.
The common features that are characteristic of TSC are:
- Brain: Many different types of non-cancerous growths (tumours or lesions) may occur in the brain. About 50% of people with TSC have an IQ in the normal range. Individuals with TSC can have clinical features such as epilepsy (seizures), learning disabilities, intellectual impairment, autism spectrum disorders and sleep disorders. There is a wide range of severity of these symptoms.
- Skin: Multiple white patches (hypopigmented macules) on the skin are often the first sign of TSC. Other skin rashes may develop with time, but none of these skin symptoms cause serious medical problems.
- Heart: Rhabdomyomas are growths that occur in the heart muscle. These often do not cause any medical problems and generally shrink with age. Very occasionally, surgery is required if the growth is blocking blood flow.
- Kidneys: Cysts and growths can occur in the kidneys. The most common type of kidney (renal) growth is called an angiomyolipoma or AML. These can sometimes cause kidney problems and there is a slightly increased risk of kidney cancer in people with TSC. A very small number of people also have another renal problem, called polycystic kidney disease as well as TSC.
- Lungs: Cysts can occur in the lungs. This is called lymphangioleiomyomatosis (LAM) of the lung. LAM may not cause any problems or may cause shortness of breath requiring medical attention. LAM affects about 30% of women with TSC, and is seen only very rarely in men.
- Eyes: Benign tumours called harmatomas can occur at the back of the eye (retina). These generally do not affect vision.
There is no cure for TSC, but the symptoms can be treated or managed. Regular surveillance to look for symptoms and early treatment are associated with better health and quality of life for people with TSC.
The genetic changes that cause Tuberous Sclerosis can lead to signs of TSC in many different organs of the body.
Signs and symptoms of tuberous sclerosis
This section provides in-depth information about the different signs and symptoms of TSC. This information should not be used as a substitute for medical advice. Readers are warned not to take any action without first seeking medical advice.
- Skin
- Brain
- Epilepsy
- Behavioural, intellectual, learning and psychiatric challenges
- Kidneys
- Heart
- Lungs
- Eyes
Skin
Nearly all people with Tuberous Sclerosis will have at least one of the signs of TSC on their skin. For many people these are one of the first signs of TSC. Signs of TSC on the skin are important for diagnosis of TSC as they comprise many of the minor and major features in the diagnostic criteria.
Although the signs of TSC on the skin are benign (non-cancerous) they can be a major concern for individuals with TSC and impact on self-esteem and social interactions.
A term used by health professionals for things to do with the skin is ‘dermatology’ and skin doctors are called dermatologists. Some treatments for the TSC skin signs can also be performed by a cosmetic surgeon.
Signs and Symptoms
Some TSC skin signs appear at birth, others develop later in childhood or even adulthood. Currently there is no way to predict how many TSC skin signs will develop during childhood, but they tend to remain stable during adulthood.
The skin signs of TSC are highly variable from one individual with TSC to the next, even within the same family. Some people have TSC skin signs that are hardly noticeable. Others may have larger TSC skin signs that cause pain or bleed easily.
The different signs of TSC on the skin are:
Hypomelanotic macules (White spots)
The white spots in TSC are called hypomelanotic macules (hypo, meaning less than normal; melanotic, referring to melanin or the pigment of skin). They are also referred to as ash-leaf spots when they are oval at one end and pointed at the other, resembling the leaf of the European mountain ash tree.
Almost all people with TSC have them; they may be present at birth and usually persist throughout life. They are often difficult to see in a newborn or in an individual with very pale skin. An ultraviolet light, called a Wood’s lamp, is used to help see them.
Hypomelanotic macules are usually the size of a thumbprint or larger. They can be scattered anywhere on the skin, but they are most common on the trunk, limbs and buttocks. If they are on the scalp, eyebrows or eyelashes they can cause a white patch of hair (poliosis).
An individual with TSC may have any number of white spots, from 1 to more than 100. In order to be useful for making a diagnosis, the individual should have 3 or more hypomelanotic macules. This is one of the major features in the diagnostic criteria for TSC (link). Fewer than 3 hypomelanotic macules do not count towards making a diagnosis because one or two hypomelanotic macules are common in the general population, occurring in about 5% of children. Sometimes numerous small macules may be present in TSC (especially on the arms and legs). These may resemble confetti, and these are a minor criterion for diagnosis.
Facial angiofibromas
Angiofibromas are found in a majority of individuals with TSC over 5 years of age. These small bumps are usually scattered on the face, especially on the nose and cheeks, and sometimes on the forehead, eyelids, and chin. They are often clustered in the grooves at the side of the nose. Angiofibromas are typically smaller than a peppercorn, but they can grow larger. They may be skin-colored, pink, or red. In darker skinned individuals they may be reddish brown or dark brown. People with TSC usually have several angiofibromas, and some individuals have hundreds.
Angiofibromas are overgrowths of normal skin cells, and they do not become cancers. They were originally called adenoma sebaceum because they were thought to be derived from sebaceous glands (grease glands) in the skin. However they have been shown in fact to be angiofibromas, composed of blood vessels (‘angio’) and fibrous tissue (‘fibroma’).
Angiofibromas may begin in early childhood as flat red “spots” on the face, or a diffuse redness of the cheeks. The redness is due to increased blood vessels in the skin. They later become elevated due to increased amounts of fibrous tissue. Fibrous tissue is similar to what is found in a scar.
Fibrous plaques
The forehead fibrous plaque is similar to an angiofibroma but is a larger area of elevated pink skin. They are usually found on the forehead, but fibrous plaques may also occur on the cheeks or scalp. Another term used for these plaques is fibrous facial plaques.
Some individuals with TSC are born with these; some develop them gradually over the first 10 years of life. After that they generally stay the same size but they can become thicker over time.
Shagreen patch
The shagreen patch is an area of thickened, elevated pebbly skin usually found on the lower back. Some people say they look like orange peel. Sometimes the shagreen patch is located elsewhere on the back or on the buttocks or upper thighs. It consists of an excess amount of fibrous tissue, similar to that found in scars.
Only about half of people with TSC have a shagreen patch and some people have many. In most people they are hidden by clothing and do not cause any problems.
Nail lesions
These are fibrous growths that are located around the fingernails or toenails . Nail lesions can be called subungual fibromas when they arise from beneath the nail and periungual fibromas when they arise from around the nail.
They do not normally affect children with TSC but often start to grow later in life. They eventually affect most adults with TSC and they can range in size from being barely detectable to almost 1 centimetre.
Ungual fibromas may distort the nail by causing a groove or by pushing the nail up from the nail bed causing infection and bleeding. On the toes, ungual fibromas can be painful when wearing shoes.
Other skin signs
There are a number of other signs of TSC that sometimes appear on the skin. Because many of these are very common in the population and they do not help doctors to diagnose TSC. Most of these will also not cause any medical symptoms.
Skin tags in TSC may be skin coloured or darker. They are common around the head and neck, armpits and groin.
Café au Lait spots, which are flat brown marks, are also seen in individuals with TSC.
Treatment
The negative effect of angiofibromas on social interactions and self-esteem can be a major concern of an individual with TSC, even when they are also living with more medically significant symptoms such as epilepsy, kidney tumours and lung problems.
Angiofibromas may also be treated for several medical reasons. The most common is bleeding and treatment lessens the likelihood of repeated episodes of bleeding.
Research in mice suggests that UV radiation may make angiofibromas worse, so daily application of a broad spectrum sunscreen is now recommended for all people with TSC. This backs up the experiences of many TSC families in Australia who report daily sunscreen use as an important part of taking care of their or their children’s skin.
A variety of surgical approaches can be used to treat angiofibromas, including the use of lasers. There are also new medicines being trialed that show positive results. You should discuss with your doctor which treatment option is right for you.
Brain
Almost all people with TSC have some signs of TSC in their brain. These signs may cause only mild symptoms in some people and severe symptoms in others.
A term used by health professionals for things to do with the brain is neurology. Doctors who specialise in the brain are called neurologists. Epileptologists are a special kind of neurologist who specialises in treating Epilepsy. A neurosurgeon may be involved to determine if brain surgery is a possible treatment for an individual with TSC.
TSC can cause the following changes in the brain:
- Epilepsy
- Behavioural, intellectual, learning and psychiatric challenges
- Cortical and subcortical tubers
- Subpendymal nodules (SENs)
- Subpendymal giant cell astrocytomas (SEGAs)
Although these changes can be called tumours, they are not cancerous.
Epilepsy
Epilepsy is a neurological condition that makes people susceptible to seizures. A seizure is a change in sensation, awareness or behaviour brought about by a brief electrical disturbance in the brain. Seizures are sometimes also called convulsions or fits. Another word for epilepsy is a seizure disorder.
Approximately 75% – 90% of individuals with TSC will have Epilepsy at some point in their life. Epilepsy can start at any age but many babies with TSC have a seizure during the first year of life.
A term used by health professionals for things to do with the brain is neurology. Doctors who specialise in the brain are called neurologists. Epileptologists are a special kind of neurologist who specialises in treating Epilepsy. An epilepsy nurse may also assist with epilepsy treatment and education
A neurosurgeon may be involved to determine if brain surgery is a possible treatment for an individual with TSC.
Epilepsy occurs in TSC is because the areas of abnormal brain development contain abnormal nerve cells. These nerve cells have abnormal bursts of electrical activity which cause the seizures.
Researchers are not sure which changes in the brain lead to seizures and why. The number and size of cortical tubers is thought to play a big part. The exact gene change that has caused TSC in the individual may also play a part. Because this is unknown, it is not possible to predict whether an individual with TSC will have seizures, what type they will be and how they should be treated.
Types of seizures may also change throughout the life of someone with TSC and epilepsy.
The different changes that occur in the brain due to TSC are covered on a separate page. Impacts of TSC and epilepsy on learning, behaviour and mental health is covered on a separate page.
- Focal, partial, generalised – what is the difference?
- Infantile spasms
- Other types of seizures
- Epilepsy syndromes
- The link between seizures and developmental delay
- Status epilepticus
Behavioural, Intellectual, Learning and Psychiatric challenges
The impacts of TSC on behaviour, learning and mental health are often the most difficult symptoms of TSC for families to cope with. It is important that families, carers, educators and health professionals are aware of these challenges; look for them in the TSC affected person at regular intervals; and implement appropriate strategies to deal with them.
The aim of understanding the different areas where a person with TSC may have difficulties is to identify the areas of strength and weakness in each individual. This is done through regular assessment and testing. After these strengths and weaknesses are identified the team of professionals can work with the family to work out what additional help may be needed to help the individual achieve their potential.
Cortical and subcortical tubers
These are found in the upper part of the brain and appear as an abnormal mass of tissue within the brain. They can also calcify or become hard. These may be large and distort the normal brain tissue. Tubers are best seen by magnetic resonance imaging (MRI).
It is thought that tubers develop along with the rest of the brain, so that the number of tubers in the brain of most individuals with TSC stays approximately the same throughout their life. This also means that tubers can be seen as early as 20 weeks’ gestation on a fetal MRI.
Tubers and the area of the brain around them, play a role in the development of seizures in TSC.
These tumours are the reason for the name Tuberous Sclerosis. “Tuber” is latin for swelling and “skleros” is greek for hard.
Subpendymal nodules (SENs)
SENs are found in about 80% of people with TSC and they are believed to not cause any symptoms. SENS are usually less than 1cm in diameter. SENs develop along with the rest of the brain but they may calcify or become hard.
If they have calcified, then SENS are visible on a Computerised Topography (CT) scan. If they have not, they may not be seen on CT images but will be seen on Magnetic Resonance Imaging (MRI).
Over time some SENs can grow to form SEGAs.
Subpendymal giant cell astrocytomas (SEGAs)
Subpendymal giant cell astrocytomas (SEGAs) are also called Subpendymal giant cell tumours (SGCTs).
The word ‘subependymal’ refers to the area below the ependyma (the membrane that lines the ventricles, or cerebrospinal fluid-filled spaces) of the brain. Giant cell refers to the very large, abnormal cells that are found with microscopic examination of the tumour. Astrocytoma refers to the type of tumor based on the most prevalent cell type.
SEGAs are usually found in the ventricles in the brain. Ventricles are natural spaces deep inside the brain filled with a clear fluid called cerebrospinal fluid (CSF). SEGAs are non-cancerous tumors, meaning they do not metastasize (spread to other parts of the brain or the body).
Even though they are not cancerous, SEGAs can be problematic because they may grow sufficiently large to block the flow of CSF within the brain, causing an increase in the pressure within the head and enlargement of the fluid-filled ventricles (a process known as hydrocephalus). This build up of pressure can result in symptoms such as vomiting, nausea, headache and changes in appetite, behavior and mood.
Up to 20% of individuals with TSC will develop a SEGA. SEGAs mostly grow from late childhood and the chance for growth greatly decreases after the mid-20s.
Typically, SEGAs are very slow growing, but occasionally they may begin to grow more rapidly. It is not known what triggers the growth of a SEGA or why some individuals with TSC have a SEGA, whereas others do not. It is also not known why only some of the subependymal nodules (SENs) grow and become SEGAs.
Surveillance
Surveillance is important because it can lead to early detection and treatment. Each person with TSC should have an individual management plan developed with their medical team that uses these guidelines as a starting point.
- An MRI study should be performed on diagnosis of TSC to get a baseline image of the brain.
- An MRI should be repeated every 1 to 3 years
- If a SEGA is found, an MRI should be repeated every 3 to 6 months to monitor growth and decide if any treatment is required.
Treatment
Tubers are sometimes treated as a part of epilepsy treatment. More information is provided here. SENs do not cause symptoms and require no treatment.
Subpendymal giant cell astrocytomas (SEGAs) require treatment when they grow large enough to potentially block the flow of fluid inside the ventricles of the brain. The main treatment options are:
- Surgery
- mTOR inhibitor medicines
Because SEGAs are a benign tumour, radiation should never be used to treat this type of brain tumour.
Kidneys
Many people with tuberous sclerosis complex (TSC) will develop some signs of TSC in their kidneys during their lifetime. However, with proper surveillance and care, most people with TSC can avoid major kidney problems. Sometimes kidney disease can be the first clue that a person has TSC; in other people it might first be detected in adulthood.
The most important things to know about TSC and the kidneys are:
- Lifelong surveillance is vital to tracking TSC growths in the kidneys and being able to offer treatment at the right time.
- New medicine for TSC kidneys has been approved and TSC experts recommend early identification and treatment of kidney angiomyolipomas (AMLs).
- Kidneys should only be removed in very extreme cases, and this should be avoided, if possible.
- The risk of kidney cancer in TSC is sometimes overstated and a TSC expert should be consulted before cancer treatment is considered.
Doctors who specialise in kidneys are called nephrologists. Another term used by health professionals for things to do with the kidneys is ‘renal’ and kidney doctors are also called renal physicians. Surgeons who operate on kidneys are called urologists.
Kidneys can be affected by TSC in a number of ways:
Angiomyolipomas (AMLs)
AMLs are benign tumours in the kidneys which are made up of blood vessels (angio), muscle (myo) and fat (lipo). The presence of fat in AMLs allows them to be distinguished from other kidney tumours by magnetic resonance imaging (MRI), computerised axial tomography (CT) scan or ultrasound imaging.
Researchers estimate that approximately 80 percent of people with TSC will develop AMLs during their lifetime. Although most people with TSC will develop AMLs, most people with TSC and AMLs won’t have major problems.
Kidney AMLs can cause concern if they start to bleed. Bleeding is rare but can be severe. A bleeding AML may cause back or abdominal pain or blood to be seen in the urine. In severe cases, AMLs can result in haemorrhage and shock and need urgent therapy, usually with embolisation. Embolisation involves a procedure that blocks off the bleeding vessel. Bleeding is very unlikely if an AML is less than 4cm in size. The risk of bleeding increases as the size of the AML increases. More research into AMLs is required to understand more about the risk of bleeding however it is generally agreed that bleeding is very unlikely if an AML is less than 4cm in size. This is why surveillance is so important.
Kidney cysts
Cysts in the kidneys are generally small and do not cause symptoms for most people with TSC. Cysts can be seen on an ultrasound.
Exactly how kidney cysts develop is not known. The TSC genes are believed to produce tumour suppressor proteins. Normally, tumour suppressor proteins prevent excess cell growth. When the TSC genes are inactivated by gene mutations, these tumour suppressor proteins are not produced and cell growth is unchecked. Cysts may, therefore, be the result of excess growth of kidney epithelial cells, which form the lining of the cysts.
Polycystic kidney disease
Up to five percent of people with TSC will also have autosomal dominant polycystic kidney disease. The gene for polycystic kidney disease is right next to the TSC2 gene. People with both diseases have a large deletion that goes across both of these adjacent genes. For these people, their severe polycystic kidney disease will usually be visible during childhood or even before birth.
Kidney cancer
People with TSC may have an increased risk of developing kidney cancer (renal cell carcinoma or RCC). The risk has been estimated to be between one and four percent and can occur at a younger age than in the general population.
These cancers are usually slow growing. If they are found early, they can be removed from the kidney, preserving kidney function. This is another reason why surveillance is important. It may be difficult to distinguish cancers from AMLs, particularly when the AMLs do not have much fat in them. Sometimes a CT scan, MRI or biopsy will be required. This can be an important time to seek the advice of the multidisciplinary team or a renal physician who is very familiar with TSC.
For a person with TSC, it is much more likely that they will develop AMLs than it is that they will develop kidney cancer.
Impaired kidney function
Most people with TSC will have normal kidney function.
Chronic kidney disease (CKD) occurs when a person’s kidneys are no longer able to perform the functions of clearing the waste products and excess fluid from the body. The kidneys have a lot of reserve capacity and some people may have a decrease in level of function but no symptoms. The risk of a person with TSC developing CKD increases with these risk factors:
- Age: the risk of CKD increases as a person with TSC gets older
- Size of AMLs: the risk of CKD increases if the person with TSC has larger AMLs
- Previous surgery to remove part of the kidney: the risk of CKD increases if previous sections of the kidney have been removed with surgery.
Only some people with TSC experience some degree of kidney impairment. Kidney failure occurs rarely in TSC except for people who also have severe polycystic kidney disease. If kidney failure occurs then dialysis or a kidney transplant are possible.
Regular surveillance and early treatment are the best tools to reduce the risk of a person with TSC experiencing impaired kidney function.
Surveillance and prevention
Surveillance is important because it can lead to early detection and treatment. Each person with TSC should have an individual management plan developed with their medical team that uses these guidelines as a starting point.
For people newly diagnosed with TSC or when TSC is suspected
- Perform magnetic resonance imaging (MRI) of the abdomen to check for possible renal angiomyolipomas or cysts.
- Measure blood pressure and glomerular filtration rate to understand kidney function.
For individuals already diagnosed with TSC
- Obtain abdominal MRI every 1-3 years to monitor renal and non-renal TSC disease progression.
- Check blood pressure and glomerular filtration rate at least annually.
The recommendation for MRIs can be difficult to follow in Australia because MRIs are not funded in our public health system. Many people with TSC in Australia have regular kidney ultrasounds, in place of MRIs, to monitor their kidneys.
A kidney ultrasound may also be recommended by your doctor because a general anaesthetic would be required or because MRI is contra-indicated for a particular individual. However, abdominal MRIs may provide more information than ultrasounds and are particularly useful if AMLs are fat-poor.
For people with TSC who have an intellectual disability or are very young, a general anaesthetic may be required before an MRI scan can be done. In these cases it is recommended to combine imaging so that multiple tests can be performed. Some people with TSC combine: abdominal MRI, brain MRI, blood tests, dental procedures and more. Although this can be very difficult to organise, people with TSC and their families find this approach minimises the impact of these tests on their lives as well as reducing risks of anaesthetic.
CT is not recommended for regular surveillance for people with TSC due to risks associated with the radiation that CT scans use. This is because people with TSC will require imaging throughout their life and repeated CT scans will possibly increase their risk of cancer later in life. However, MRI is not useful for detecting lung disease so women with TSC will have CT scans performed. Read more in our information page about TSC and the lungs.
Monitoring blood pressure is important because high blood pressure, or hypertension, can accelerate a loss of kidney function. Blood tests to measure glomerular filtration rate find out how well the kidneys are working.
People with TSC should aim for a healthy diet and lifestyle to ease the burden on the kidneys and avoid high blood pressure. Kidney Health Australia has more information about keeping kidneys healthy.
Treatment
Many people with TSC will not require any treatment for their kidneys. Kidney cysts and small AMLs do not require treatment.
High blood pressure should be treated to reduce the stress on the kidneys. Polycystic kidney disease and chronic kidney disease should be treated as they are for the non-TSC population.
Angiomyolipomas (AMLs) should be treated if they bleed or if they are large and/or growing.
1. Treatment for a bleeding AML
Embolisation is a procedure that blocks the blood vessels that supply the AMLs. This stops the bleeding and causes the AML to shrink. It is very important that this is combined with corticosteroids for seven days to reduce the risk of post-embolisation syndrome.
2. Treatment for a large and growing AML
mTOR inhibitor medicines are recommended to treat AMLs that are larger than three centimetres in diameter and continue to grow. The medicine approved in Australia for this purpose is Everolimus (Afinitor) and it is available on the Pharmaceutical Benefits Scheme (PBS). You should discuss this medicine with your doctor if you have an AML that is 3cm or larger in size. Your doctor can consider your personal circumstances including age, growth rate and other aspects of your health to make a decision about whether this medicine is a suitable treatment.
This medicine is known to reduce the size of AMLs and we know that smaller AMLs have a lower risk of bleeding. We don’t yet know whether treatment with mTOR inhibitors reduces the risk of a person with TSC developing chronic kidney disease. More research is being done to understand their role in keeping TSC kidneys healthier for longer.
Alternative treatment options for AMLs include embolisation and surgery. Surgery should aim to preserve as much kidney tissue as possible and partial and full nephrectomy should be avoided. While there are some people with TSC who have had successful kidney transplants, the aim of regular surveillance and appropriate intervention is to keep the kidney as healthy as possible.
Heart
At birth or in infancy, approximately 50% of individuals with TSC have at least one tumour in their heart. These benign (non-cancerous) tumours are called a cardiac rhabdomyoma do not usually cause any symptoms. Most rhabdomyomas decrease in size or disappear within the first 1-2 years of life.
The term used by health professionals for things to do with the heart is cardiac. Doctors who specialise in children’s hearts are called paediatric cardiologists.
Signs and symptoms
Rhabdomyoma
Cardiac rhabdomyomas may be detected during pregnancy using standard antenatal ultrasound but may not appear until the second half of pregnancy. They may be single or multiple and are usually the first clinical clue that the baby has TSC.
They are non-cancerous tumours, which means they do not metastasize, or spread throughout the body. Although it is not clear why, rhabdomyomas typically regress, or shrink, during childhood. Less commonly they can remain stable, newly appear or even grow.
Symptoms
- Most individuals with TSC who have cardiac rhabdomyomas will have no symptoms.
- For a smaller number of individuals, symptoms depend on the number of tumours and their size and location within the heart.
- Larger tumours can obstruct blood flow through the heart and lead to heart failure or cyanosis (blue discolouration of the skin due to insufficient blood oxygenation).
- An abnormal heart rhythm, called an arrhythmia can be caused when a tumour is close to the heart’s electrical conduction system.
- Poor heart muscle function, or cardiac myopathy, can be caused by replacement of muscle in the heart ventricles with tumour tissue.
- A small minority of TSC patients with rhabdomyomas have a heart murmur.
Other
Some individuals with TSC also have vascular abnormalities, which means abnormal blood vessels. These can lead to high blood pressure and very rarely other complications. High blood pressure can also be caused by kidney symptoms
Treatment
Because most rhabdomyomas regress in size, watchful monitoring may be all that is necessary in individuals who have rhabdomyomas that are not interfering with the blood flow or rhythm of the heart.
Individuals with significant arrhythmias may require treatment such as medication. There have been reports of successful use of mTOR inhibitor medicines to treat large rhabdomyomas in infancy.
Lungs
Some people with TSC, especially women, may have signs of TSC in their lungs. The most common sign is lymphangioleiomyomatosis (LAM). Most of these people will not have any symptoms but it is still recommended for all women to have a scan of their chest to look for signs of TSC in their lungs.
Terms used by health professionals for things to do with the lungs are respiratory and pulmonary. Doctors who specialise in lungs and lung disease can be called respiratory physicians.
Signs and Symptoms
Signs of TSC in the lungs are much more commonly found in women, usually only in adulthood. There have only been a small number of cases of TSC affecting the lungs in men.
The lungs can be affected by TSC in two different ways:
- Lymphangioleiomyomatosis (LAM)
- Multifocal micronodular pneumocyte hyperplasia (MMPH)
People with TSC can be affected with many other lung diseases, just like everyone else in the community. These include infections such as influenza and pneumonia as well as asthma.
Treatment
Severe LAM is a serious illness, but treatment is available and there is a large amount of research being done to identify and trial new treatments for LAM.
Most treatments for LAM are aimed at easing symptoms and preventing complications. The main treatments are:
- mTOR inhibitor medicines
- Medicines to improve air flow in the lungs and reduce wheezing
- Oxygen therapy
- Procedures to remove fluid from the chest or abdomen and stop it from building up again
- Hormone therapy
- Lung transplantation
- Each individual with LAM will work with their doctor to decide the best treatments for them.
Vaccinations for influenza and pneumonia should also be considered because these infections can be very serious in individuals with LAM. Supplemental oxygen during air travel and avoiding low oxygen environment such as high altitude and unpressurised aeroplane cabins may also be recommended for some individuals with LAM.
Eyes
There are a variety of signs of TSC that can involve the eyes and approximately 50% of people with TSC have some signs of TSC in their eyes. Loss of vision is not common in TSC.
Terms used by health professionals for things to do with the eyes are ophthalmic and ocular. An ophthalmologist is a medical doctor who specialises in the eyes. The retina is the part of the eye that transmits what is seen by the eye to the brain via the optic nerve.
There are many different signs of TSC that can occur in and around the eyes. Many of these do not cause major problems but can be helpful when trying to diagnose TSC.
Even for a doctor, it can be very difficult to tell if an abnormality in the eyes is caused by TSC or some other cause.
- Behavioural difficulties
- Developmental delay
- Autism Spectrum Disorders
- Attention Deficit Hyperactivity Disorder (ADHD)
- Mood and Anxiety Disorders
- Other Psychiatric Disorders
- Sleep difficulties
- Intellectual ability, learning and academic skills
- What causes these symptoms of TSC?
- Kidney Cysts and Polycystic Kidney Disease
- Angiomyolipomas (AMLs)
- Kidney Cancer
- Impaired kidney function
- Rhabdomyoma
- Symptoms
- Other
- Lymphangioleiomyomatosis (LAM)
- Multifocal micronodular pneumocyte hyperplasia (MMPH)
- Retinal Harmatomas
- Retinal Hypopigmented Lesions
- Angiofibromas around the eyes
- Vision problems caused by intellectual impairment
- Vision problems caused by epilepsy treatment
- Other signs
Genetic testing for tuberous sclerosis
Clinical geneticists are medical doctors that specialise in genetics and genetic diseases. Genetic counsellors are health professionals that are trained in both counselling and medical genetics. Genetic counselling is a process that can help the whole family understand how TSC is inherited and to make decisions about management and reproduction.
Important points
- At present it is impossible to predict who will remain only mildly affected and who will be more severely affected by TSC. Even members of the same family can be affected differently.
- There are two genes that are known to be associated with TSC. These are called TSC1 and TSC2. People with TSC – regardless of the severity of their symptoms – all have a variation in one of their TSC genes that makes it faulty.
- The pattern of inheritance of the faulty gene causing TSC is described as autosomal dominant inheritance.
- When a parent has a faulty TSC gene copy they have a 1 in 2 (50%) chance in each pregnancy of having a child with TSC.
- In about 30% of the cases, TSC is inherited from an affected parent. In the remaining 70% of cases, the person with TSC is the first in the family with the condition. This is likely to have occurred due to a change in one copy of a TSC gene during the formation of the egg or sperm, during conception or shortly after conception (a spontaneous mutation that occurred for unknown reasons).
- The diagnosis of TSC is based on clinical features and genetic testing is usually not required. However, genetic testing for changes in the TSC genes can be helpful in some situations, such as testing a baby in pregnancy for TSC where one of the parents is affected. It is highly recommended that testing be discussed in the context of genetic counselling.
What causes Tuberous Sclerosis Complex (TSC)?
Genes, chromosomes and genetic conditions
In all the cells of our body, our genes are found on chromosomes (long strings of genes). Most of our chromosomes (and therefore our genes) come in pairs – one of each copy from each parent. The only exception is the sex chromosomes, which determine whether we are male or female. We have many thousands of genes that provide information for our body to grow, develop and remain healthy. The gene sends messages to the cell to make important chemical products such as proteins.
Everyone has variations in the information in their genes, which is why we are all unique. Variations can either be harmless or at times, can cause a gene to be faulty. Variations that make a gene faulty are called mutations. The information contained in the faulty gene, and its product, is impaired.
Faulty genes do not work as they should in the body and are unable to provide the correct information to our cells. A fault in either of two different genes – one called TSC1 and one called TSC2 – can cause TSC. These genes make proteins that make sure cells only grow as fast as they need to. TSC1 has the instructions for creating a protein called hamartin and TSC2 has the instructions for a protein called tuberin. If these proteins are not being produced correctly, some cells grow in an uncontrolled way forming the tumours seen in people with TSC.
When we know that a person has TSC, we can look for the responsible gene change in the TSC1 and TSC2 genes. We find that a TSC2 gene change is found in about one quarter and a TSC1 gene change is found in about two-thirds of people with TSC. Current genetic testing technologies do not find a gene change responsible for TSC in all people with a diagnosis of TSC. For more information, see “What does it mean if my DNA test did not find a mutation?”
What is the pattern of inheritance in TSC families?
A faulty TSC gene can either be passed down (inherited) from a parent or may occur as a new faulty gene just before or after conception. Once a faulty gene is present in an individual’s egg or sperm cells, it can be passed on to future generations. This is referred to as genetic inheritance.
Two factors influence the pattern of inheritance of a faulty TSC gene.
The pattern of inheritance in families of the faulty gene causing TSC is therefore described as autosomal dominant inheritance
When a parent has TSC
In about 30% of the cases, TSC is inherited from an affected parent. When one of the parents has TSC due to the faulty TSC gene, there are four possible combinations of the genetic information that can be passed on to their children. This means that, in each pregnancy:
- There are 2 chances in 4 (1 chance in 2, or 50%) that their child will inherit the faulty TSC gene and will therefore be affected by TSC.
- There are 2 chances in 4 (1 chance in 2, or 50%) that their child will inherit the working TSC gene from the affected parent. In this case, the child will not develop TSC and cannot pass on the faulty TSC gene copy to any of their children.
While the picture shows the father as the parent with the faulty TSC gene copy, the same situation would arise if the mother had the faulty TSC gene copy. TSC usually affects men and women equally.
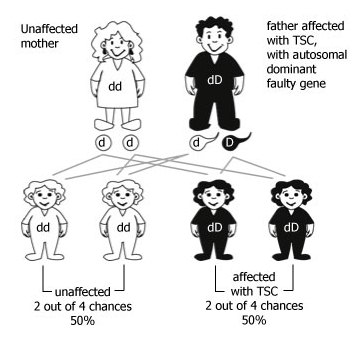
Figure 1: Autosomal dominant inheritance when one parent has a faulty copy of one TSC gene. The faulty TSC gene copy is represented by ‘D’; the working copy by ‘d’
When neither parent has TSC but they have a child with the condition
In approximately 70% of cases, the person with TSC is the first in the family with the condition. In these people, the condition resulted from a change that occurred in one copy of the TSC1 or TSC2 genes during formation of the egg or sperm, during conception or shortly after. These changes that make one of the TSC gene copies faulty are called ‘spontaneous mutations’. Spontaneous mutations are not caused by any action of the parents but arise by chance, as a new change.
Once a person has TSC s/he may potentially pass on the faulty gene copy to his/her children as described earlier.
If the TSC gene became faulty shortly after conception, not all of the baby’s cells may contain the gene variation; this individual is said to be ‘mosaic’ for the faulty TSC gene. They may experience milder symptoms because the faulty gene may not be present in all of the organs usually affected in TSC. The faulty gene might not be in all the egg or sperm cells of an individual with mosaic TSC. Their chance of passing on the faulty gene is therefore less than 50%.
If a child of a parent who is mosaic for TSC inherits the faulty TSC gene copy, they may be more severely affected by TSC than their parent. This is because the child has the faulty gene in all the cells of their body, while their parent only has the faulty gene in some cells. That child also has a 50% risk of passing on the faulty gene copy to his or her children.
Because of the possibility that an unaffected parent of a child with TSC is mosaic for the faulty TSC gene, the chance of having another child affected by TSC is estimated between 1% and 3%. The chance that a spontaneous mutation in the TSC gene would happen again in further pregnancies is low.
TSC does not ‘skip generations’. However sometimes the features of the condition are so subtle that individuals do not realise they have TSC. This is the reason that the guidelines for people newly diagnosed with TSC are to review the newly diagnosed individual’s nearest three generations (siblings, parents, and either children or grandparents). An assessment by a skin specialist (dermatologist), eye doctor (ophthalmologist) and genetics doctor (clinical geneticist) may be useful in either confirming or ruling out that someone is affected with TSC.
Can a person have a test for a faulty TSC gene?
Even though TSC is a genetic condition, genetic testing is not needed to diagnose the condition. Most people with TSC will have enough physical signs of the condition for a specialist to diagnose them with confidence.
Genetic testing is available but is complex, time consuming and expensive. Testing the TSC1 and TSC2 genes will find a mutation in only about 80% of affected individuals. This is because there may be other genes that cause TSC that have not yet been identified. Alternatively, it is also possible that current genetic testing techniques are not yet sensitive enough to pick up all the variations that can cause TSC.
Genetic testing can however be helpful in some situations such as:
- Confirming a possible diagnosis of TSC where there aren’t enough clinical features for a specialist to make a confident diagnosis.
- Testing a baby in pregnancy (prenatal testing) or an embryo in IVF (pre-implantation genetic diagnosis) for TSC where one of the parents is affected
- Testing parents, sisters or brothers of someone with TSC to establish whether or not they have TSC
Genetic testing may also be helpful in the future as new treatments for TSC may be specific to either the TSC1 or TSC2 gene.
It is highly recommended that testing be discussed in the context of genetic counselling.
Prepared by: A/Prof Kristine Barlow-Stewart & Ron Fleischer, The Centre for Genetics Education; Clare Stuart, The Australasian Tuberous Sclerosis Society. This page has been adapted from the Genetics Fact Sheet that has been co-authored by The Australasian Tuberous Sclerosis Society and The Centre for Genetics Education.
When is genetic testing for TSC useful?
Genetic testing is not required in every individual with TSC. However it may be helpful in a number of situations.
When an individual is suspected to have TSC but does not have enough signs of the disease to meet the full diagnostic criteria, a genetic test may be useful to confirm or rule out TSC.
The 2012 revision of the diagnostic criteria for TSC included genetic testing criteria for the first time. This means that it may be possible to diagnose TSC if a mutation is found on the TSC1 or TSC2 gene that is known to cause TSC in other individuals. Read more about diagnostic criteria for TSC.
Genetic testing may also be useful to test for TSC in family members. This includes when a child in a family is diagnosed with TSC and the parents wish to have more children in the future. Siblings of an individual with TSC may also find genetic testing for TSC helpful in planning their families.
When an individual with TSC wishes to plan for their own family, genetic testing may allow them to use prenatal testing to avoid passing on TSC to their children.
What is involved in having a genetic test?
A small amount of blood is drawn (usually one or two tubes), and the blood is shipped to the laboratory performing the genetic testing by the laboratory performing the blood draw.
Genetic testing for TSC is best done in the context of genetic counselling. Genetic counselling provides education, information and support.
Related
ncG1vNJzZmilqZm%2Fb6%2FOpmWarV%2BgtqW%2FjK2cnqajYrWmrcutn2ispZeys7vUrGSsm5yav7C%2FyKxm